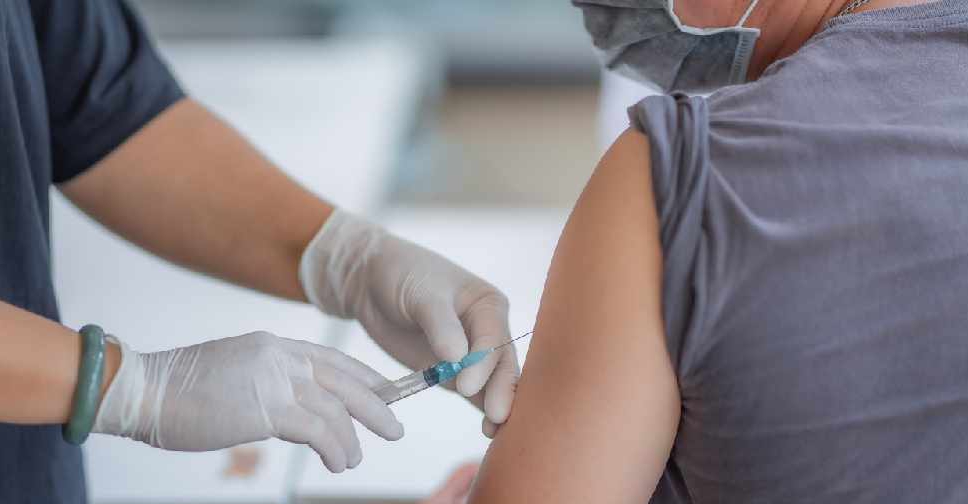
South Korean drugmaker Celltrion Inc said on Monday it has received regulatory approval for Phase 3 clinical trials of an experimental COVID-19 treatment.
The approval comes as the company plans to seek conditional approval for its antibody-drug, CT-P59, for emergency use by the end of this year.
The treatment, the most advanced antibody-drug in terms of research in South Korea, is directed against the surface of the virus and designed to block it from locking on to human cells.
The third stage trials will be conducted on some 1,000 asymptomatic coronavirus patients and those who have come into close contact with COVID-19 patients in Korea, Celltrion said in a statement.
The Ministry of Food and Drug Safety recently approved a Phase 2/3 study on patients with mild and moderate cases of COVID-19, Lee Sang-joon, Celltrion's senior executive vice president, told Reuters.
Celltrion began commercial production of the drug in September - likely to amount to around 1 million doses - in anticipation of demand in both domestic and overseas markets.
In July, Celltrion separately launched overseas human trials of its treatment in Britain.




 US House rejects war powers resolution, backs Trump on Iran war
US House rejects war powers resolution, backs Trump on Iran war
 GCC and EU ministers urge immediate halt to Iranian attacks
GCC and EU ministers urge immediate halt to Iranian attacks
 Trump wants say on Iran's next leader
Trump wants say on Iran's next leader
 British PM Starmer to send four Typhoon jets to Qatar
British PM Starmer to send four Typhoon jets to Qatar
 Bombing of Tehran intensifies as war enters day six
Bombing of Tehran intensifies as war enters day six
 Azerbaijan vows to respond after four injured by Iranian drones
Azerbaijan vows to respond after four injured by Iranian drones
 72 killed in Israeli attacks on Lebanon as it warns residents to leave south
72 killed in Israeli attacks on Lebanon as it warns residents to leave south
 Nepal goes to the polls; voters seek change after youth-led protests
Nepal goes to the polls; voters seek change after youth-led protests




