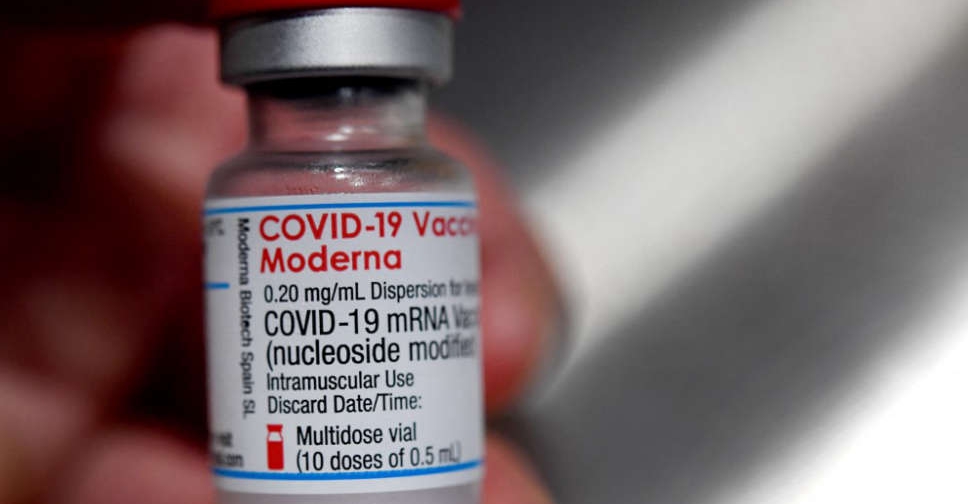
The UAE's Ministry of Health and Prevention has announced the registration of Moderna's COVID-19 vaccine for emergency use.
It's the fifth COVID-19 vaccine to be approved for use in the country.
The decision was made following the completion of clinical trials and a strict assessment, as well as the approval of the US Food and Drug Administration (FDA).
Dr. Mohamed Salim Al-Olama, Undersecretary of the Mohap, highlighted that the move is crucial to accelerate the pace of recovery and community immunization.
The decision enables health authorities to import the vaccine after fulfilling shipping-related safety and efficacy standards, Dr Amin Hussein Al Amiri added.
Currently, four vaccines are available in the UAE - Pfizer-BioNTech, Oxford AstraZeneca, Sputnik V and Sinopharm.



 H.H. Sheikh Mohammed honours winners of 'Great Arab Minds'
H.H. Sheikh Mohammed honours winners of 'Great Arab Minds'
 Dubai trials shared school transport service with luxury SUVs
Dubai trials shared school transport service with luxury SUVs
 UAE pledges $1.5 million to support UN Human Rights programme
UAE pledges $1.5 million to support UN Human Rights programme
 UAE President holds talks with Turkish Foreign Minister
UAE President holds talks with Turkish Foreign Minister
 Dubai to organise world’s largest virtual sign language class
Dubai to organise world’s largest virtual sign language class
 UAE expresses solidarity with Thailand over deadly train accident
UAE expresses solidarity with Thailand over deadly train accident
 UAE activates automatic degree recognition for 34 universities
UAE activates automatic degree recognition for 34 universities
 World Safety Summit launches in Dubai
World Safety Summit launches in Dubai




