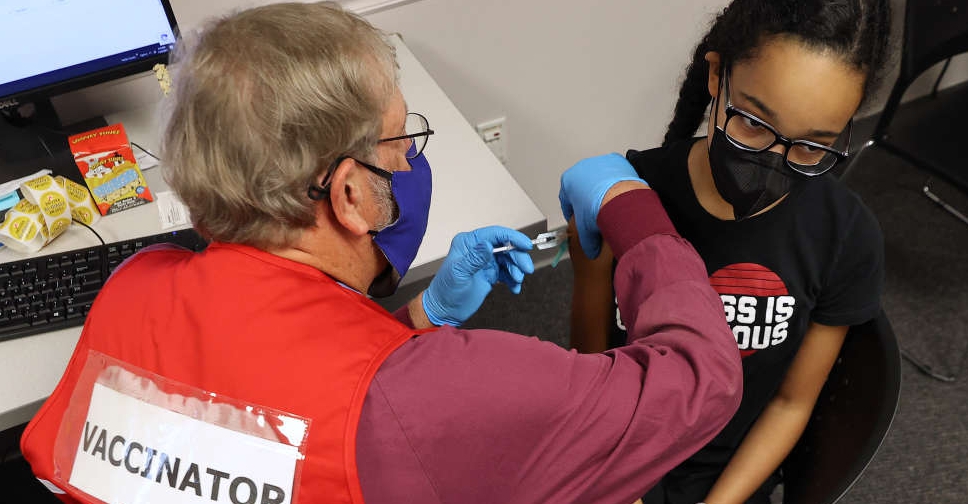
The US Food and Drug Administration (FDA) has authorised the use of a third dose of the Pfizer and BioNTech COVID-19 vaccine for children aged between 12 and 15 years.
The agency has also narrowed the time for all booster shots to 5 months from 6 months after primary doses.
Meanwhile, a third shot has been authorised in children aged 5 through 11 years who are immunocompromised.
The FDA said it reviewed published data and real world evidence on the safety of booster doses provided by the Israeli Ministry of Health including data from over 6,300 individuals 12-to-15 years of age who received a Pfizer shot.
Global COVID-19 cases are surging due to the Omicron variant and health authorities have warned that its extremely high transmissibility could overwhelm many health systems.
Laboratory tests have shown that two doses of the Pfizer-BioNTech and Moderna vaccines generate low immune responses against Omicron, while boosters appear to be protective against the highly-mutated variant.




 US House rejects war powers resolution, backs Trump on Iran war
US House rejects war powers resolution, backs Trump on Iran war
 GCC and EU ministers urge immediate halt to Iranian attacks
GCC and EU ministers urge immediate halt to Iranian attacks
 Trump wants say on Iran's next leader
Trump wants say on Iran's next leader
 British PM Starmer to send four Typhoon jets to Qatar
British PM Starmer to send four Typhoon jets to Qatar
 Bombing of Tehran intensifies as war enters day six
Bombing of Tehran intensifies as war enters day six
 Azerbaijan vows to respond after four injured by Iranian drones
Azerbaijan vows to respond after four injured by Iranian drones
 72 killed in Israeli attacks on Lebanon as it warns residents to leave south
72 killed in Israeli attacks on Lebanon as it warns residents to leave south
 Nepal goes to the polls; voters seek change after youth-led protests
Nepal goes to the polls; voters seek change after youth-led protests




