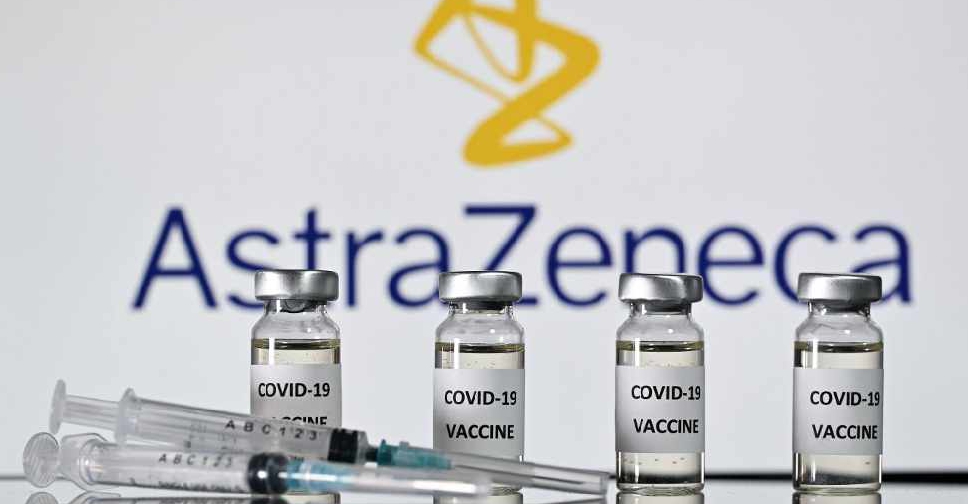
The University of Oxford has launched a study to assess the safety and immune response of the COVID-19 vaccine it has developed with AstraZeneca Plc in children for the first time, it said on Saturday.
The new mid-stage trial will determine whether the vaccine is effective on people between the ages of 6 and 17, according to an emailed statement from the university.
Around 300 volunteers will be enrolled and first inoculations are expected this month, Oxford said.
The two-dose Oxford/AstraZeneca vaccine has been hailed as a "vaccine for the world" because it is cheaper and easier to distribute than some rivals.
AstraZeneca has a target to produce 3 billion doses this year and aims to produce over 200 million doses per month by April.




 Iran names new supreme leader
Iran names new supreme leader
 GCC permanent representatives meet UN chief
GCC permanent representatives meet UN chief
 Qatar arrests 313 individuals for spreading misinformation
Qatar arrests 313 individuals for spreading misinformation
 Saudi Arabia renews condemnation of Iranian attacks
Saudi Arabia renews condemnation of Iranian attacks
 Two dead, 12 injured after projectile hits Saudi residential location
Two dead, 12 injured after projectile hits Saudi residential location
 Bahrain destroys 95 missiles, 164 drones since start of Iranian attacks
Bahrain destroys 95 missiles, 164 drones since start of Iranian attacks
 Qatar Airways to run limited flights to Doha as airspace closure continues
Qatar Airways to run limited flights to Doha as airspace closure continues
 Three injuries and material damage to a university building in Bahrain
Three injuries and material damage to a university building in Bahrain




