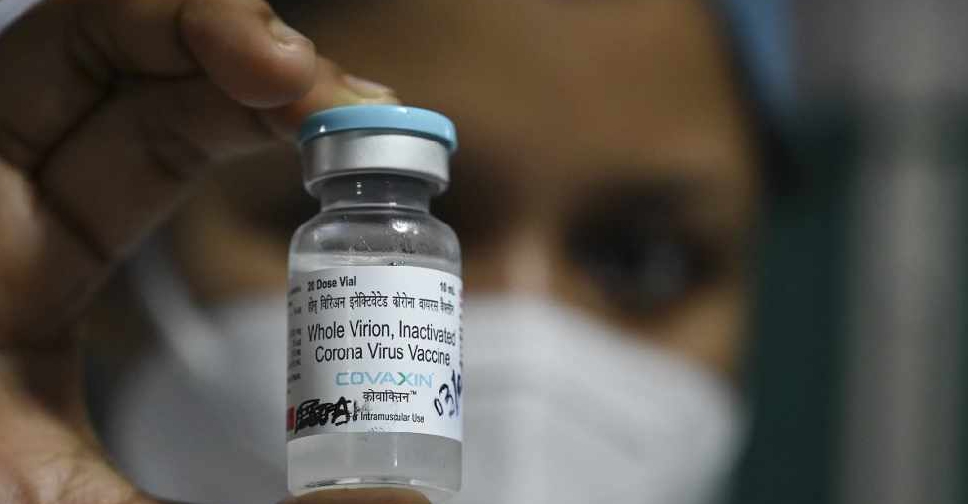
Bharat Biotech's homegrown COVID-19 vaccine has shown an interim vaccine efficacy of 81% in late-stage clinical trials, the Indian company said on Wednesday.
The interim analysis was based on 43 recorded cases of COVID-19 in the trial of 25,800 participants, conducted in partnership with the Indian government's medical research body.
Thirty-six of the 43 cases were recorded in participants who received a placebo, compared with seven cases in people who were given the Bharat Biotech vaccine, pointing to an efficacy rate of 80.6%, the company said.
India had approved the vaccine, branded COVAXIN, in January without late-stage efficacy data, raising questions about its effectiveness.
India's vaccination drive, currently underway, includes COVAXIN and a vaccine developed by Oxford University and AstraZeneca.
Earlier this week, Indian Prime Minister Narendra Modi was inoculated with the first dose of COVAXIN.




 Israeli airforce pounds Beirut, Lebanon death toll rises
Israeli airforce pounds Beirut, Lebanon death toll rises
 Hezbollah warns Israeli residents to evacuate towns near border
Hezbollah warns Israeli residents to evacuate towns near border
 US House rejects war powers resolution, backs Trump on Iran war
US House rejects war powers resolution, backs Trump on Iran war
 Saudi Arabia, Qatar, Kuwait intercept drones targeting territory
Saudi Arabia, Qatar, Kuwait intercept drones targeting territory
 GCC and EU ministers urge immediate halt to Iranian attacks
GCC and EU ministers urge immediate halt to Iranian attacks
 India's tech state Karnataka bans social media for children under 16
India's tech state Karnataka bans social media for children under 16
 Indonesia says it will withdraw from Board of Peace if it does not benefit Palestinians
Indonesia says it will withdraw from Board of Peace if it does not benefit Palestinians
 Trump wants say on Iran's next leader
Trump wants say on Iran's next leader




