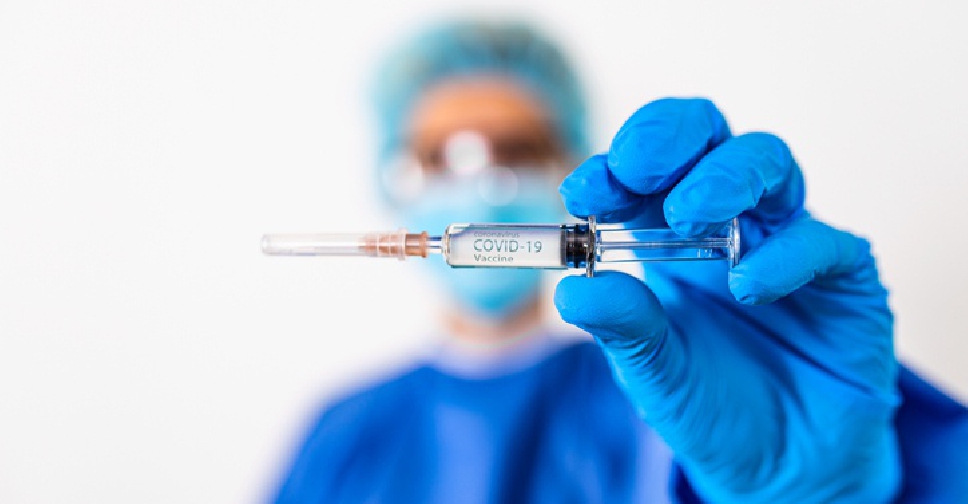
India's drug regulator on Tuesday allowed vaccine maker Serum Institute to enrol children aged 7-11 years for its COVID-19 vaccine trial as the country prepares to protect kids from the coronavirus.
It has already administered more than 870 million doses to adults among its population of nearly 1.4 billion.
"After detailed deliberation, the committee recommended for allowing enrolment of subjects of 7 to 11 years of age group as per the protocol," a subject expert panel of the Central Drugs Standard Control Organisation said.
Serum Institute is already conducting a trial of its COVID-19 vaccine, a domestically produced version of US drugmaker Novavax's shot, in the 12-17 age group and has presented safety data for an initial 100 participants.
So far, only drugmaker Zydus Cadila's DNA COVID-19 vaccine has received emergency use approval in India to be used in adults and children aged 12 years and above.




 Explosion hits US embassy in Oslo, causing minor damage, thick smoke
Explosion hits US embassy in Oslo, causing minor damage, thick smoke
 Four killed in Beirut hotel strike, Israel says it targeted Iranian commanders
Four killed in Beirut hotel strike, Israel says it targeted Iranian commanders
 Drone attacks cause major fire at Kuwait International Airport
Drone attacks cause major fire at Kuwait International Airport
 Israel warns Lebanon of 'heavy price' as week's death toll nears 300
Israel warns Lebanon of 'heavy price' as week's death toll nears 300
 Trump rejects settling war with Iran
Trump rejects settling war with Iran
 Qatar intercepts 6 ballistic missiles, 2 cruise missiles
Qatar intercepts 6 ballistic missiles, 2 cruise missiles
 Trump tells Britain he does not need its help to win Iran war
Trump tells Britain he does not need its help to win Iran war
 Israeli settler fatally shoots Palestinian in West Bank
Israeli settler fatally shoots Palestinian in West Bank




