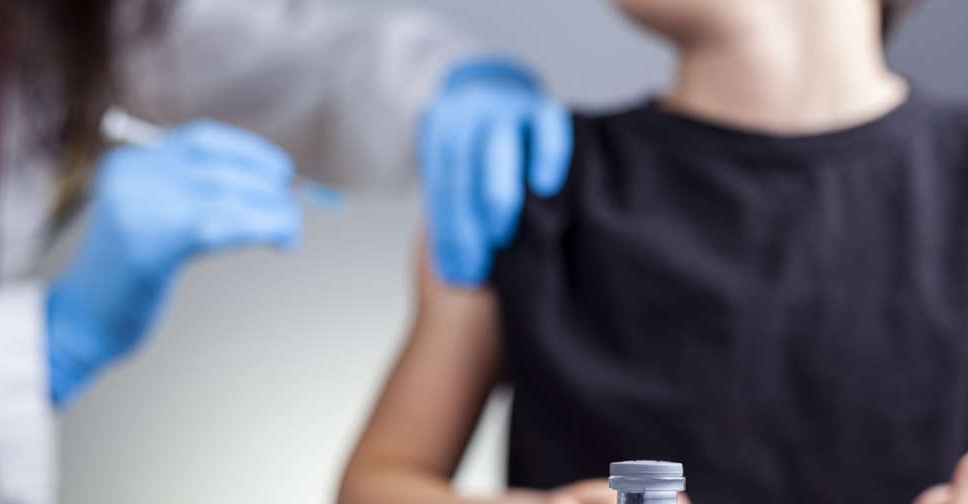
The UAE's Ministry of Health and Prevention has given emergency approval for Sinopharm COVID-19 vaccine for children aged three and above.
In a tweet, the National Emergency Crisis and Disasters Management Authority (NCEMA) said the decision was made after "the recent clinical trials and extensive evaluations, which are in line with the approved regulations".
Ministry of Health approves use of #Sinopharm vaccine for 3-17 age group #WamNews pic.twitter.com/yq8snmccJu
— WAM English (@WAMNEWS_ENG) August 2, 2021
MoHAP announced the provision of Sinopharm vaccine for the age group 3 - 17 The decision comes after clinical trials and extensive evaluations and is based on the emergency use authorization and local evaluations which are in line with the approved regulations.#TogetherWeRecover pic.twitter.com/4Y3VEMMW8V
— NCEMA UAE (@NCEMAUAE) August 2, 2021
It comes after authorities in Abu Dhabi achieved the target of vaccinating 900 children as part of a trial to study the effectiveness of the Sinopharm COVID-19 vaccine in young people.
The Department of Health Abu Dhabi (DoH) said the study examined the vaccine’s effectiveness in reducing the infection rate and severity of symptoms among target groups.
Its preliminary results will be announced at a later stage.




 Dubai unveils region's first integrated recreational vehicle route
Dubai unveils region's first integrated recreational vehicle route
 RTA deploys drones to inspect Dubai Metro tunnels
RTA deploys drones to inspect Dubai Metro tunnels
 Emergency calls surge amid weather fluctuations in Dubai
Emergency calls surge amid weather fluctuations in Dubai
 UAE President discusses strategic partnership with Elon Musk
UAE President discusses strategic partnership with Elon Musk
 Abu Dhabi warns community on fire hazards during winter
Abu Dhabi warns community on fire hazards during winter
 UAE, UNHCR sign deal to support Sudan conflict response
UAE, UNHCR sign deal to support Sudan conflict response
 H.H. Sheikh Hamdan highlights UAE's tech progress with Elon Musk
H.H. Sheikh Hamdan highlights UAE's tech progress with Elon Musk
 UAE President meets French counterpart in Abu Dhabi
UAE President meets French counterpart in Abu Dhabi




