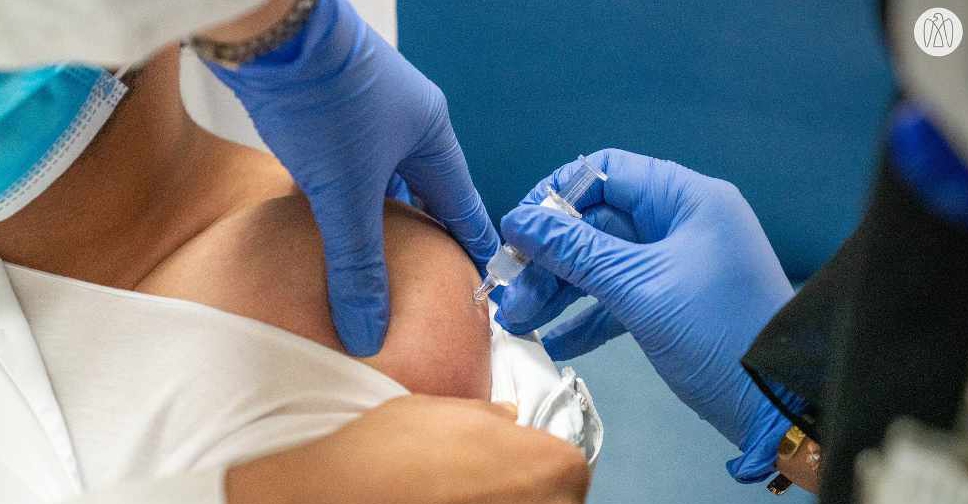
The trials for a COVID-19 inactivated vaccine in the UAE have reached a key milestone.
Five thousand volunteers have been vaccinated since the testing programme began on July 16.
More than 80 different nationalities are currently participating in the third phase of the clinical trial that is open to all UAE residents as part of the #4Humanity programme.
Participants can register online, while there's also a dedicated centre each in Abu Dhabi and Sharjah to receive volunteers.
Up to 15,000 volunteers are required in order to conclude the scientific research phase.
G42 Healthcare is managing the overall clinical trial process under the supervision of the Department of Health Abu Dhabi (DoH) and in coordination with Abu Dhabi Health Services Company, SEHA.
The inactivated vaccine used in the trials has been developed by China's Sinopharm CNBG, and has shown promising results during the earlier Phase I and Phase II trials.
The #4Humanity COVID-19 inactivated vaccine trials today reached a significant milestone with the 5,000th volunteer vaccinated, testimony to the national spirit of volunteerism and strength of the healthcare system that has enabled the speed of this achievement. pic.twitter.com/qhY01uzPQy
— مكتب أبوظبي الإعلامي (@ADMediaOffice) August 6, 2020




 Dubai mandates front number plates for delivery bikes
Dubai mandates front number plates for delivery bikes
 UAE, European Commission Presidents explore closer ties
UAE, European Commission Presidents explore closer ties
 UAE, Cyprus Presidents discuss enhancing strategic partnership
UAE, Cyprus Presidents discuss enhancing strategic partnership
 Emirates, Dubai Humanitarian launch airbridge for Sri Lanka cyclone victims
Emirates, Dubai Humanitarian launch airbridge for Sri Lanka cyclone victims
 RTA chalks out route map for Dubai Metro Blue Line
RTA chalks out route map for Dubai Metro Blue Line
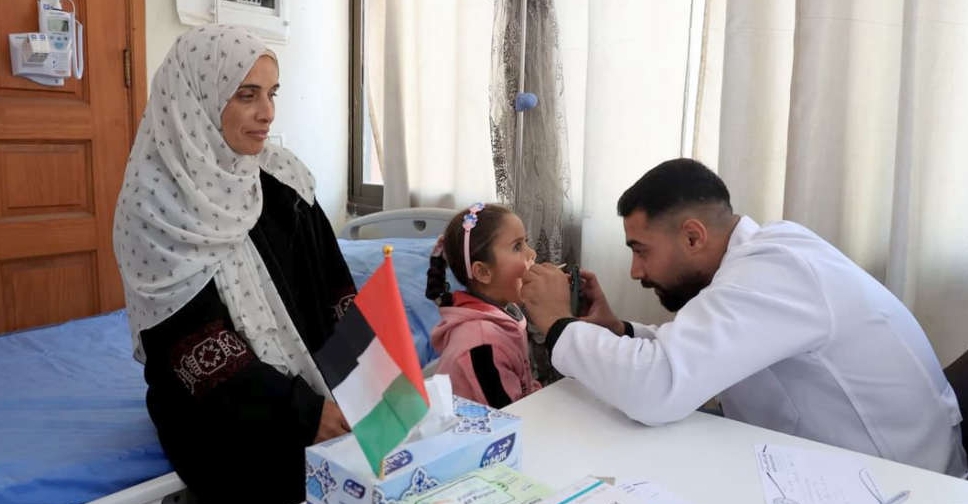 UAE opens Emirates Medical Centre to serve patients in Gaza
UAE opens Emirates Medical Centre to serve patients in Gaza
 UAE President receives official welcome at Presidential Palace in Nicosia
UAE President receives official welcome at Presidential Palace in Nicosia
 Rain hits parts of UAE: Dubai Police issues public safety SMS alerts
Rain hits parts of UAE: Dubai Police issues public safety SMS alerts




