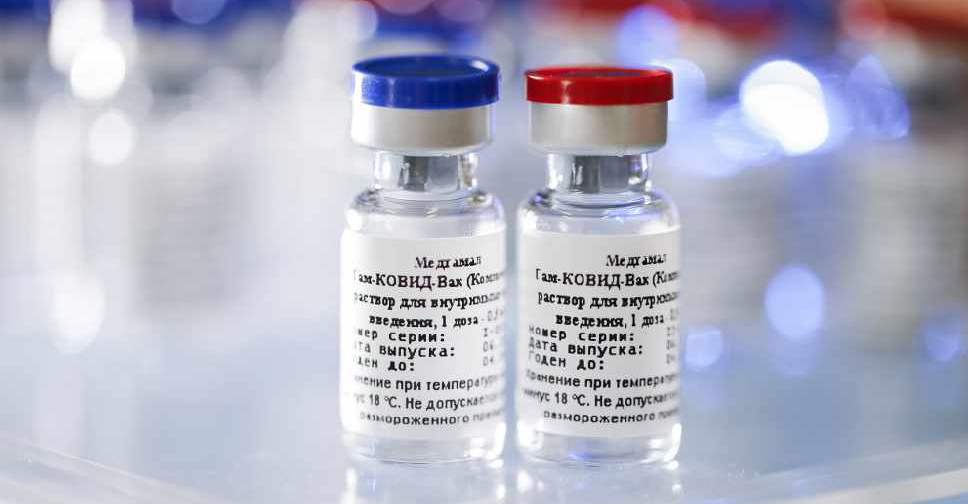
The volunteer programme for Phase 3 clinical trials of a Russan COVID-19 vaccine has been launched in the UAE.
Five-hundred volunteers from Abu Dhabi will initially be invited to participate in the campaign called Vaccine for Victory.
Eligible participants must be Abu Dhabi residents aged at least 18 years; not have been infected with COVID-19 or suffered any communicable or respiratory diseases in the past 14 days.
Also, they must not have participated in any other COVID-19 vaccination trial.
Volunteers will receive two doses of the vaccine, administered 20 days apart, and will be regularly monitored through visits and teleconsultations.
The volunteer programme for Phase III clinical trials of the Russian human adenovirus-based vaccine has been launched under the #VaccineforVictory campaign. 500 volunteers from Abu Dhabi will initially be invited to participate. pic.twitter.com/CUXEQyXGlx
— مكتب أبوظبي الإعلامي (@ADMediaOffice) December 7, 2020
The trials in the UAE are facilitated through a partnership between the Russian Direct Investment Fund (RDIF), Russia’s sovereign wealth fund, and Abu Dhabi-based Aurugulf Health Investment.
They will be conducted by Abu Dhabi's Department of Health (DoH), with joint supervision by the UAE Ministry of Health and Prevention, while medical protocols will be handled by the Abu Dhabi Health Services Company, SEHA.
The human adenovirus-based vaccine was developed by the Gamaleya National Research Institute of Epidemiology and Microbiology of the Ministry of Health of the Russian Federation.




 NCM forecasts cloudy weather, rain in parts of UAE
NCM forecasts cloudy weather, rain in parts of UAE
 Sri Lankan President thanks UAE for flood relief efforts
Sri Lankan President thanks UAE for flood relief efforts
 Dubai’s beaches, parks reopen as weather improves
Dubai’s beaches, parks reopen as weather improves
 Some Dubai flights cancelled due to adverse weather conditions
Some Dubai flights cancelled due to adverse weather conditions
 Dubai deploys special patrols, emergency crew to monitor traffic during rains
Dubai deploys special patrols, emergency crew to monitor traffic during rains
 Dubai private schools to shorten Friday hours from January
Dubai private schools to shorten Friday hours from January
 UAE leaders congratulate Morocco on FIFA Arab Cup win
UAE leaders congratulate Morocco on FIFA Arab Cup win
 UAE ministry urges remote work for private sector in weather-hit areas
UAE ministry urges remote work for private sector in weather-hit areas




