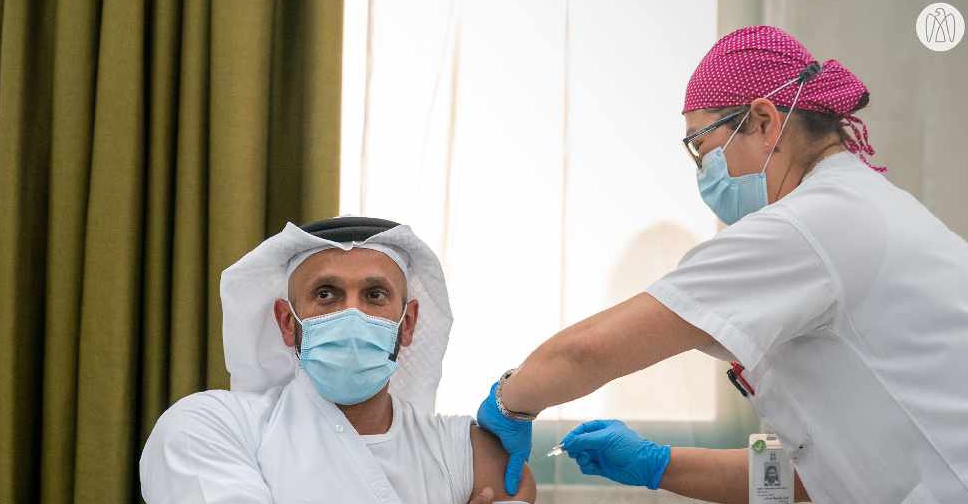
The third phase of clinical trials for a COVID-19 vaccine has begun in Abu Dhabi.
The chairman of the Department of Health- Abu Dhabi (DoH), Sheikh Abdullah bin Mohammed Al Hamed is the first person to participate in the trial.
Dr. Jamal Al Kaabi, Acting Undersecretary of DoH will be the second in line.
The trials are being performed by Abu Dhabi-based G42 Healthcare in cooperation with China's Sinopharm CNBG, under the administration of the DoH and the UAE's Ministry of Health.
عبد الله بن محمد آل حامد، رئيس دائرة الصحة بأبوظبي، أول متطوع يشارك في التجارب السريرية التي تجري في #أبوظبي للمرحلة الثالثة من اللقاح غير النشط لوباء كوفيد-19، يليه الدكتور جمال الكعبي، وكيل دائرة الصحة بالإنابة، وهو الأمر الذي يعكس التزام الإمارة بإيجاد علاج عالمي للوباء. pic.twitter.com/TX6AJPxJh3
— مكتب أبوظبي الإعلامي (@ADMediaOffice) July 16, 2020
It's the world’s first WHO-listed phase III clinical trial of the COVID-19 inactivated vaccine.
The world’s first @WHO-listed phase III clinical trial of the Covid-19 inactivated vaccine has begun in #AbuDhabi, with @DoHSocial Chairman, Sheikh Abdullah bin Mohammed Al Hamed the 1st to participate in the trial, followed by Acting Undersecretary Dr. Jamal Al Kaabi. pic.twitter.com/gEIFgcSFHC
— مكتب أبوظبي الإعلامي (@ADMediaOffice) July 16, 2020
The UAE's plans to launch the trials were announced last month, while earlier this week, Minister of Health and Prevention, Dr. Abdul Rahman Al Owais revealed more than 15,000 volunteers will be participating.




 UAE President meets French counterpart in Abu Dhabi
UAE President meets French counterpart in Abu Dhabi
 UAE, UNHCR sign deal to support Sudan conflict response
UAE, UNHCR sign deal to support Sudan conflict response
 H.H. Sheikh Mohammed congratulates 'Great Arab Minds Economics' winner
H.H. Sheikh Mohammed congratulates 'Great Arab Minds Economics' winner
 New Dubai Trade Centre bridges slash journey times to two minutes
New Dubai Trade Centre bridges slash journey times to two minutes
 UAE welcomes statements by US Secretary of State on Sudan
UAE welcomes statements by US Secretary of State on Sudan
 UAE approves Itvisma gene therapy to treat spinal muscular atrophy
UAE approves Itvisma gene therapy to treat spinal muscular atrophy
 Cloudy weather, rain forecast for parts of UAE
Cloudy weather, rain forecast for parts of UAE
 Sri Lankan President thanks UAE for flood relief efforts
Sri Lankan President thanks UAE for flood relief efforts




