Volunteers who wish to participate in the world’s first phase III clinical trial of the COVID-19 inactivated vaccine in Abu Dhabi can now register their details.
Up to 15,000 volunteers are being sought for the trial with a minimum of 5,000 required.
Places are open to people between the ages of 18-60 who are living in Abu Dhabi or Al Ain.
All applicants will be required to undergo a medical check-up to assess their eligibility.
The trials are being performed by Abu Dhabi-based G42 Healthcare in cooperation with China's Sinopharm CNBG, under the administration of the Department of Health- Abu Dhabi (DoH) and the UAE's Ministry of Health.
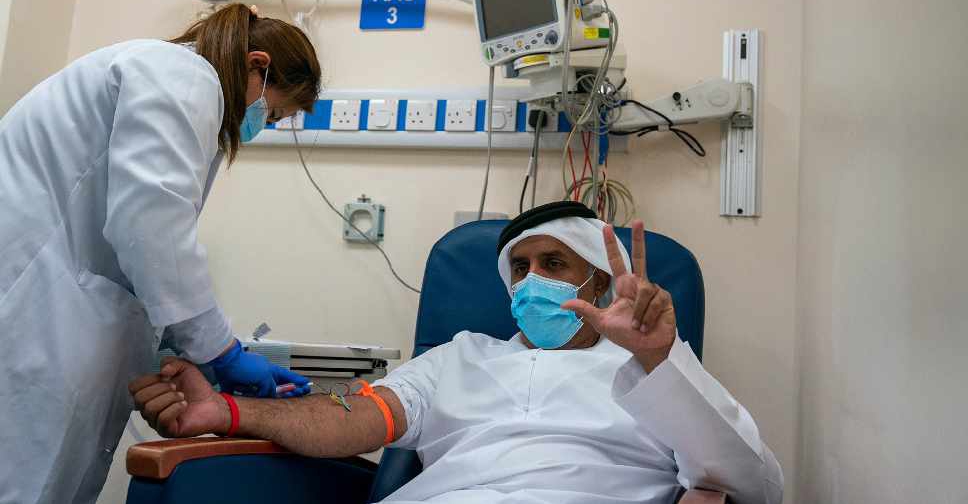
The chairman of the DoH, Sheikh Abdullah bin Mohammed Al Hamed, is the first person to participate in the trial, followed by the Acting Undersecretary of DoH, Dr. Jamal Al Kaabi.
For more information, and to register to take part, visit http://4humanity.ae.
Chairman of @DoHSocial Abdullah bin Mohammed Al Hamed discusses the significance of the Covid-19 inactivated vaccine's phase III clinical trials in #AbuDhabi, and encourages members of the community to become part of this historic moment by volunteering to participate.
— مكتب أبوظبي الإعلامي (@ADMediaOffice) July 17, 2020




 Some Dubai flights cancelled due to adverse weather conditions
Some Dubai flights cancelled due to adverse weather conditions
 Dubai deploys special patrols, emergency crew to monitor traffic during rains
Dubai deploys special patrols, emergency crew to monitor traffic during rains
 Dubai private schools to shorten Friday hours from January
Dubai private schools to shorten Friday hours from January
 UAE leaders congratulate Morocco on FIFA Arab Cup win
UAE leaders congratulate Morocco on FIFA Arab Cup win
 UAE ministry urges remote work for private sector in weather-hit areas
UAE ministry urges remote work for private sector in weather-hit areas
 UAE completes loading aid ship with 10 million meals for Gaza
UAE completes loading aid ship with 10 million meals for Gaza
 Dubai declares remote work for govt. staff, private sector urged to follow suit
Dubai declares remote work for govt. staff, private sector urged to follow suit
 Dubai carriers issue travel advisory amid fluctuating weather
Dubai carriers issue travel advisory amid fluctuating weather




