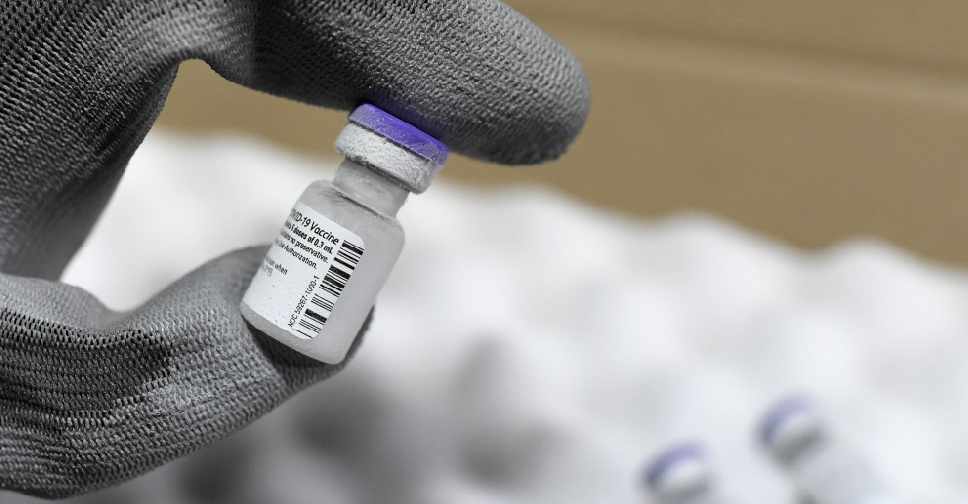
The Philippines is evaluating the emergency use of Pfizer's COVID-19 vaccine, the presidential spokesman said on Saturday.
Pfizer was the first company to seek the Philippine regulator's approval for emergency use of its coronavirus vaccine, Harry Roque, spokesman of President Rodrigo Duterte, said in a statement.
It will take the food and drugs agency 21 days to evaluate and approve the vaccine, he said, adding that inoculation would start as soon as stocks become available.
The Philippines has the second highest number of COVID-19 infections and deaths in Southeast Asia, next to Indonesia.




 WHO says first five patients evacuated via Gaza's Rafah crossing
WHO says first five patients evacuated via Gaza's Rafah crossing
 Russia pounds Ukrainian energy in diplomatic snub, Zelenskyy says
Russia pounds Ukrainian energy in diplomatic snub, Zelenskyy says
 Paris prosecutor's cybercrime unit searches X office
Paris prosecutor's cybercrime unit searches X office
 Spain to ban social media access for children under 16
Spain to ban social media access for children under 16
 Clintons agree to testify in Epstein congressional probe ahead of contempt vote
Clintons agree to testify in Epstein congressional probe ahead of contempt vote
 Air India checking fuel switches on its Boeing Dreamliners, memo says
Air India checking fuel switches on its Boeing Dreamliners, memo says
 Trump seeks $1 billion from Harvard University in damages
Trump seeks $1 billion from Harvard University in damages
 US to cut tariffs on India to 18%, India agrees to end Russian oil purchases
US to cut tariffs on India to 18%, India agrees to end Russian oil purchases




